

Relative Strength Of Common Directing Groups In Palladium Catalyzed Aromatic C H Activation Sciencedirect
Is It True That Fluorine Is Always The Strongest Electron Withdrawing Group Ewg Due To Fluorine S Unrivaled Electronegativity Quora

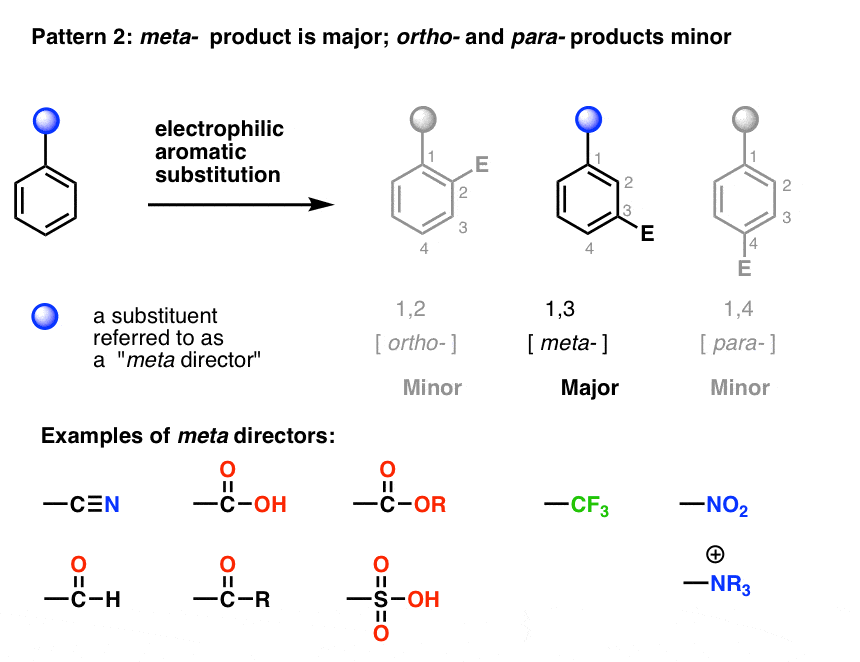
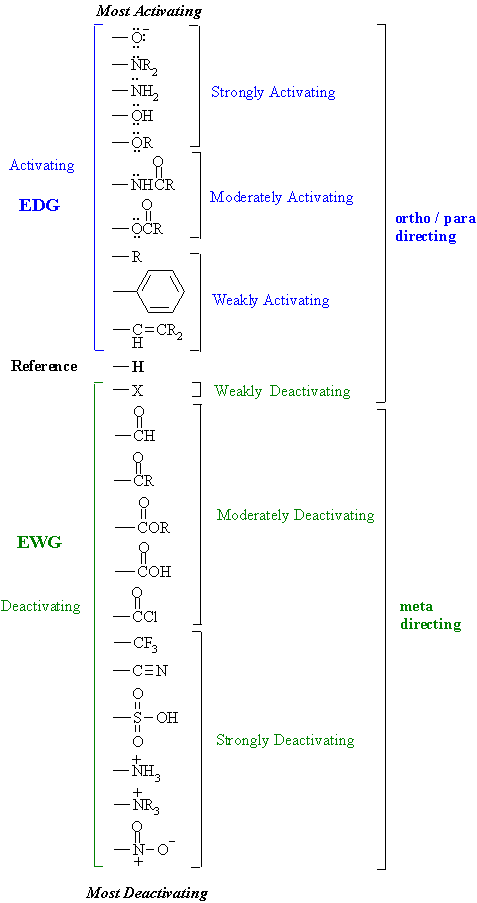
Why Are Ortho And Para Directors In Aromatic Rings Electron Donating Whereas Meta Directors Are Electron Withdrawing Quora
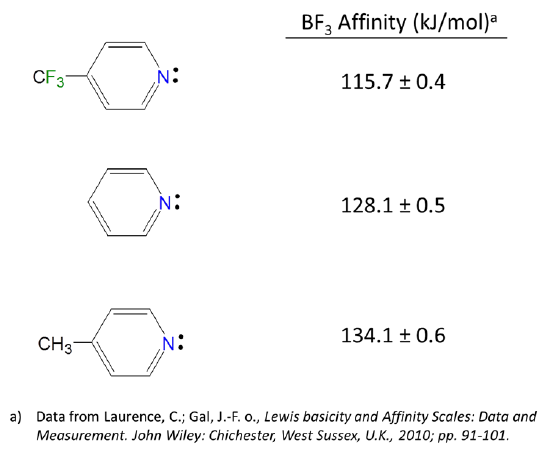
6 4 2 All Other Things Being Equal Electron Withdrawing Groups Tend To Make Lewis Acids Stronger And Bases Weaker While Electron Donating Groups Tend To Make Lewis Bases Stronger And Acids Weaker

Relative Strength Of Common Directing Groups In Palladium Catalyzed Aromatic C H Activation Sciencedirect

Relative Strength Of Common Directing Groups In Palladium Catalyzed Aromatic C H Activation Sciencedirect
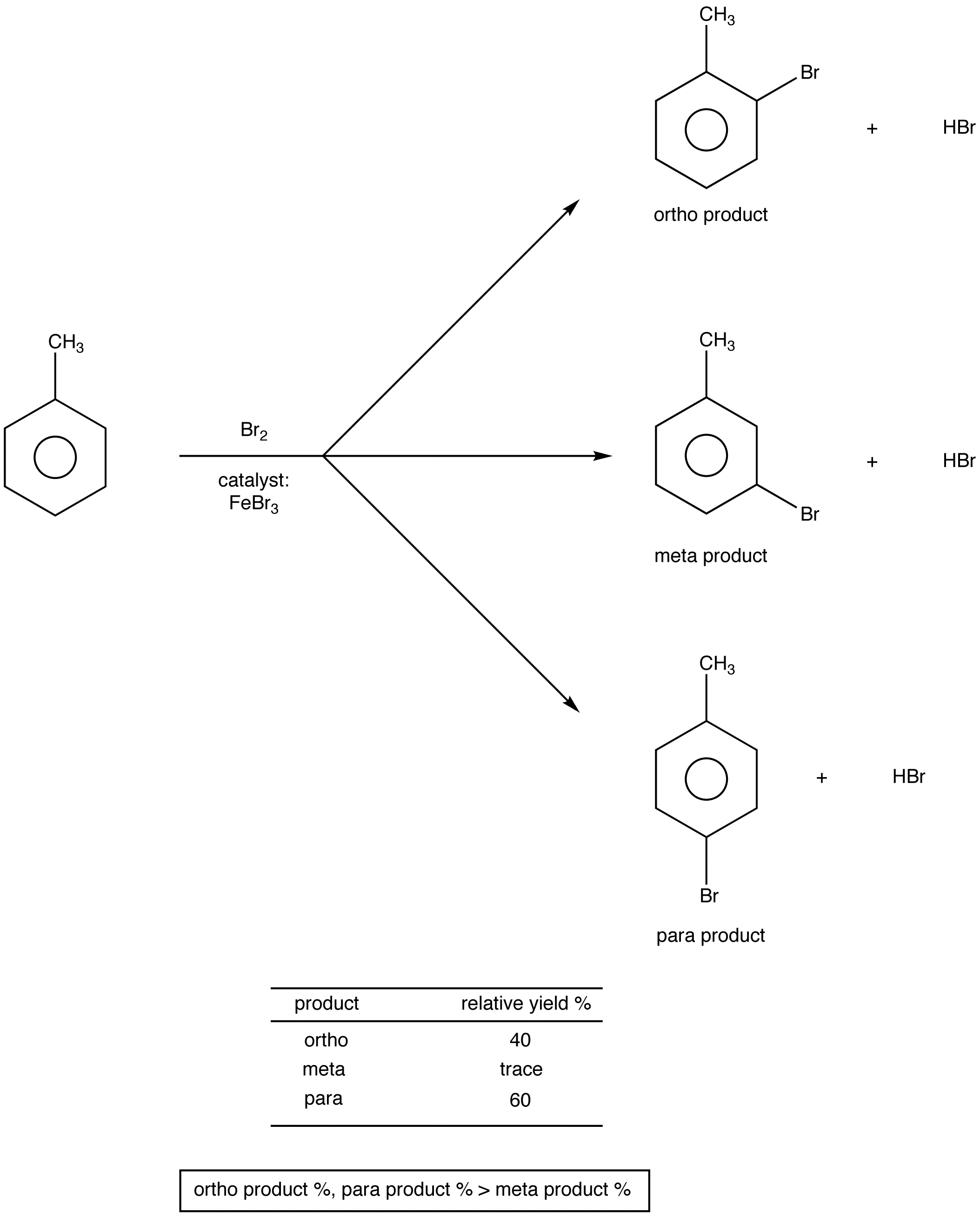
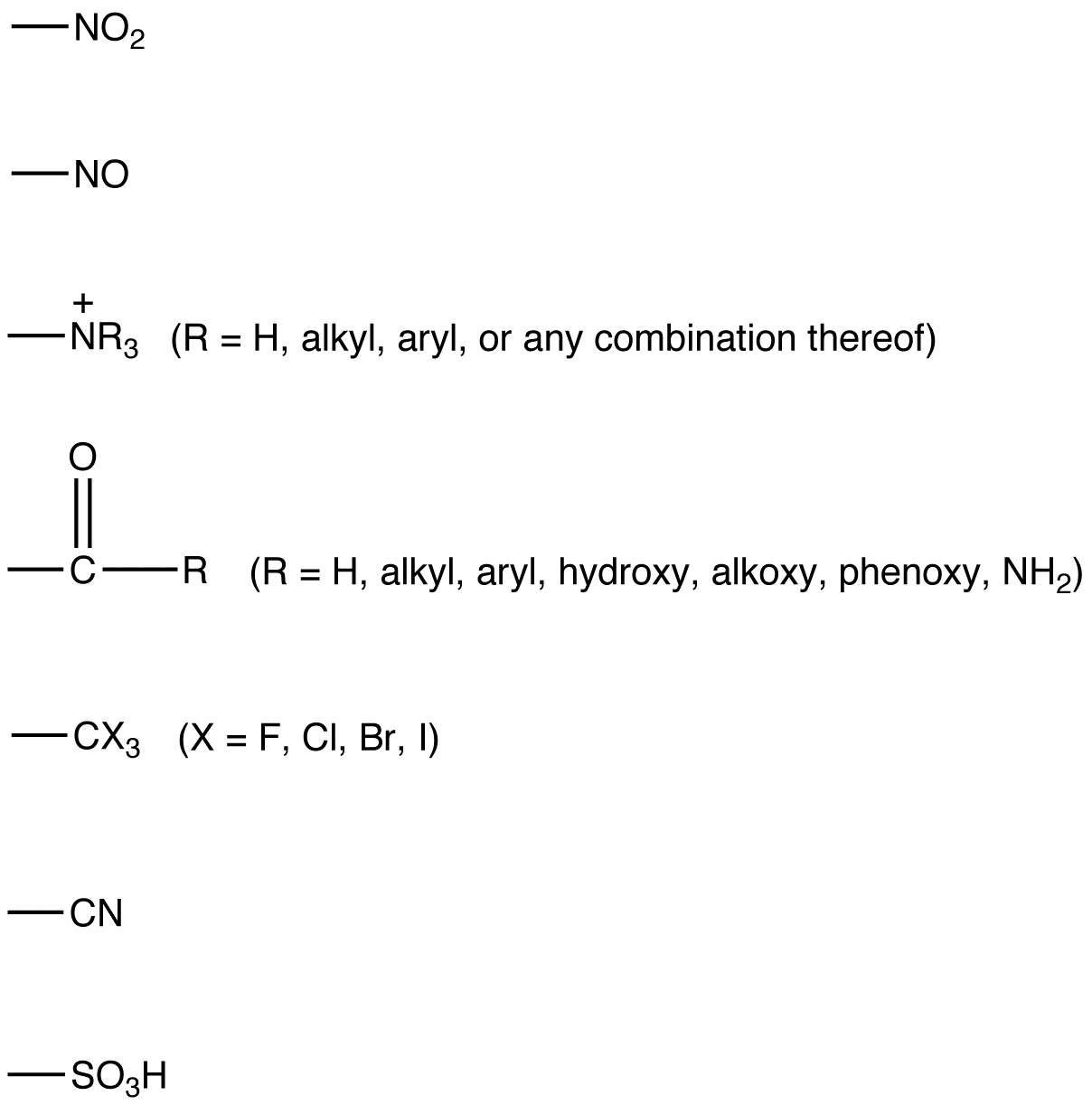
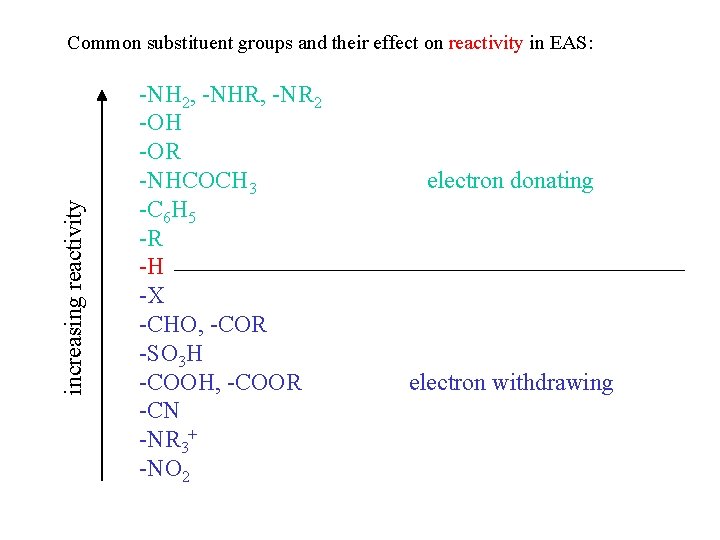


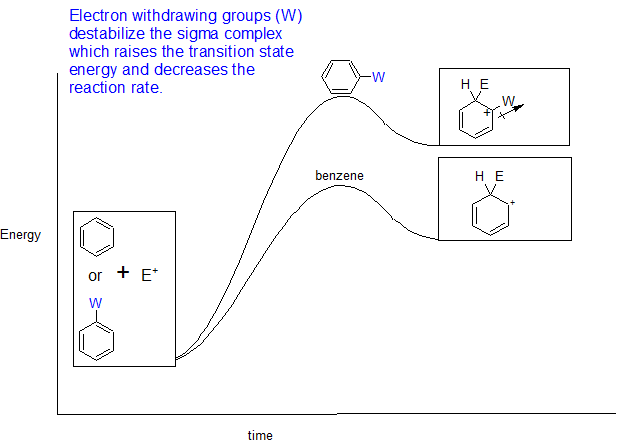
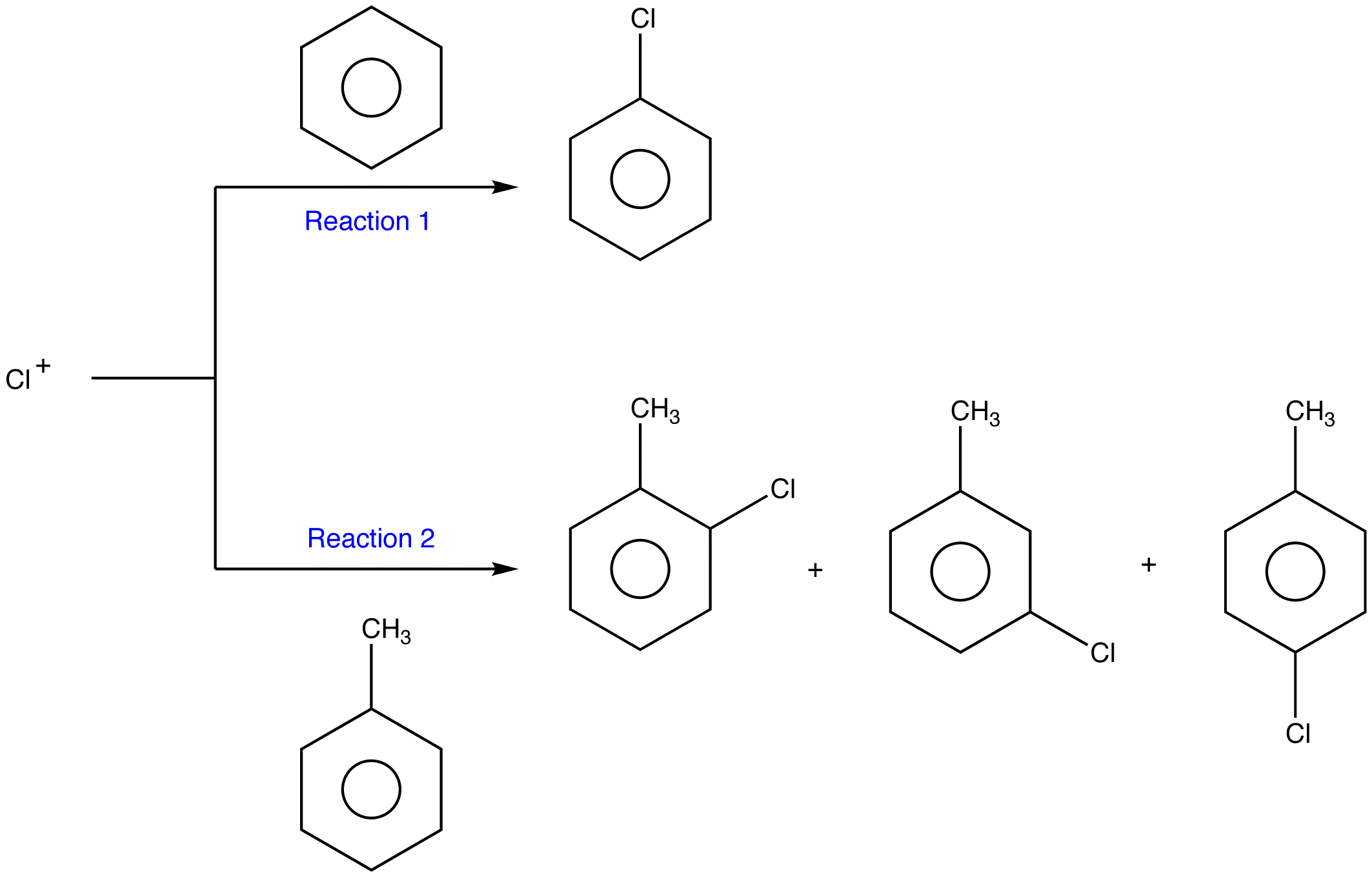
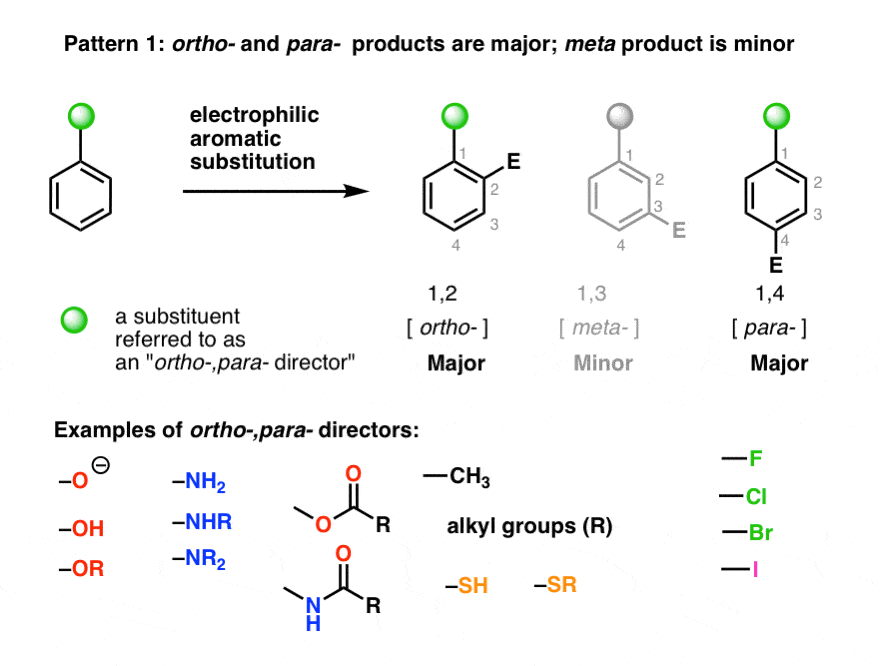

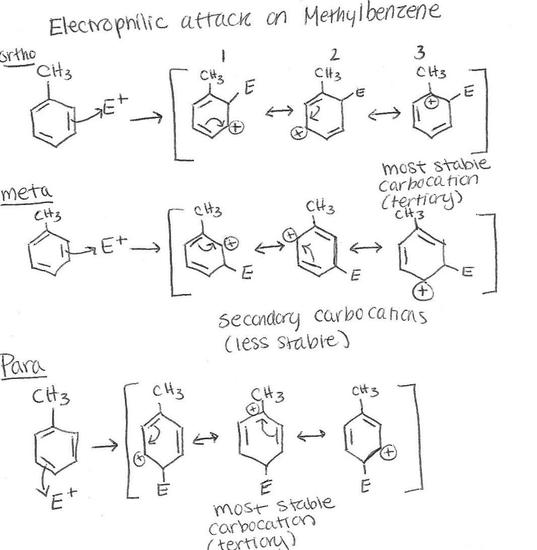


.png?revision=1)
No comments:
Post a Comment