They will also be introduced to concepts like Thomson Rutherford and Bohr atomic models. In CoH2O62 the central metal atom Co is in 2 oxidation state.

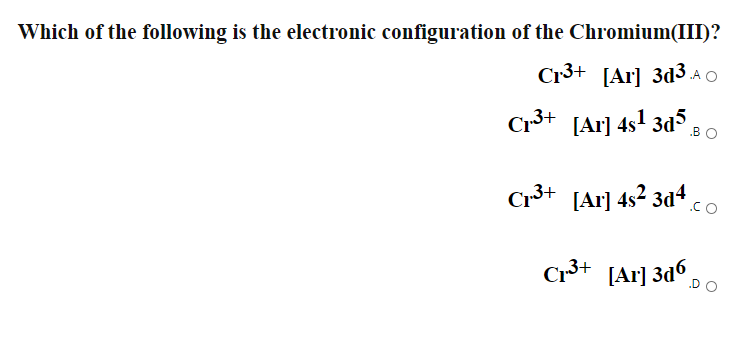
Give The Electronic Configuration Of Mn2 And Cr3 Chemistry Some Basic Concepts Of Chemistry 13094403 Meritnation Com

13 Write The Complete Electron Configuration And Indicate The Number Of Unpaired Electrons For Each Of The Followin Homeworklib
View Answer The activation energy for the gas phase.

Cr3 electron configuration.
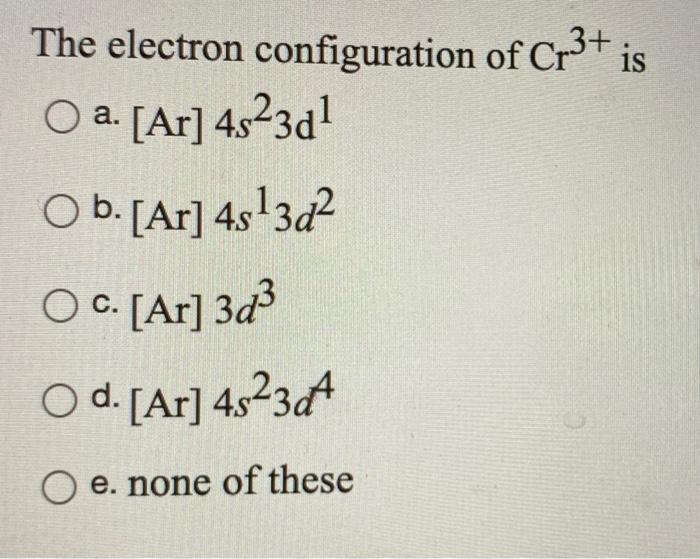
Cr3 Electronic configuration a 1s2 2s2 2p6 3s2 3p6 3d10 b 1s2 2s2 2p6 3s2 3p6 c 1s2 2s2 2p6 3s2 3p6 3d10 4s1 d 1s2 2s2 2p6.
Students will also get to know about how electron proton and neutron were discovered and learn their characteristics.
As the central metal atom is d²sp³ hybridized.
If an element with the valence configuration 4s23d7 loses 3 electrons these electrons would be removed from the Blank subshells.
After gaining energy E the vacant 4s 4p and 3d orbitals make d²sp³ hybridization and 6 lone pairs from 6 water molecules.

How Is The Electron Configuration Of Cr3 1s2 2s2 2 6 3s2 3p6 3d5 4s1 Ar 3d5 4s1 Ar 3d3 Home Work Help Learn Cbse Forum

Give The Electronic Configuration Of Mn2 And Cr3 Chemistry Some Basic Concepts Of Chemistry 13094403 Meritnation Com

Lo 1 5 The Student Is Able To Explain The Distribution Of Electrons In An Atom Or Ion Based Upon Data Sec 7 12 Lo 1 6 The Student Is Able To Analyze Ppt Download

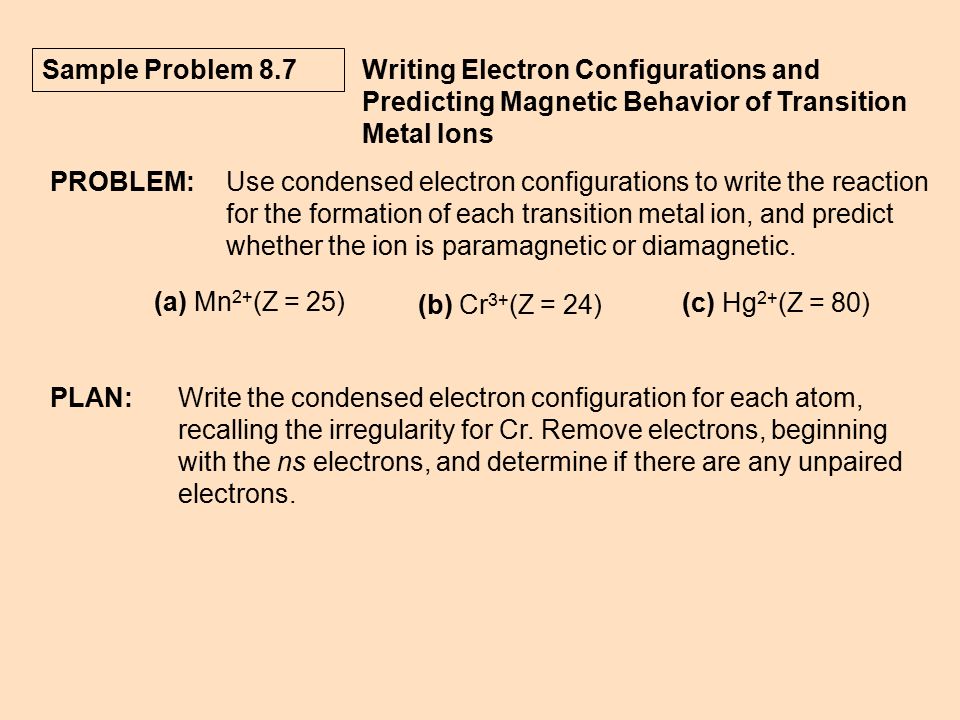
For Each Atom Ion Provide The Condensed Ground State Electron Configurations The Box Diagram Of The Valence Electrons And The Number Of Unpaired Electrons S N Cr Cr3 Fe2 N3 As Study Com

6 Give The Electron Configuration Use Inert Gas Symbol For Core Electrons And Orbital Diagram For Valence Electrons Homeworklib

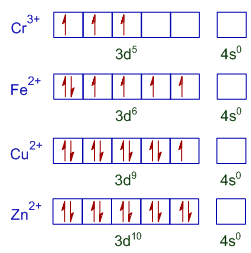
The D Electron Configurations Of Cr3 Mn2 Fe2 And Co2 Are D4 D5 D6 And D7 Respectively Which One Of The Following Will Exhibit Minimum Paramagnetic Behaviour At No Cr 24

Write Down Electronic Configuration Of Cr3 And Cu2 Ions Calculate The Number Of Unpaired Electrons Brainly In

3 12 And 4 4 16 And 5 48 The Pair Of Ions Having Same Electronic Configuration Is 1 Cr3 Fe3 3 Fe3 Co3 2 Fe3 Mn2 4 Sc3 Cr3












No comments:
Post a Comment